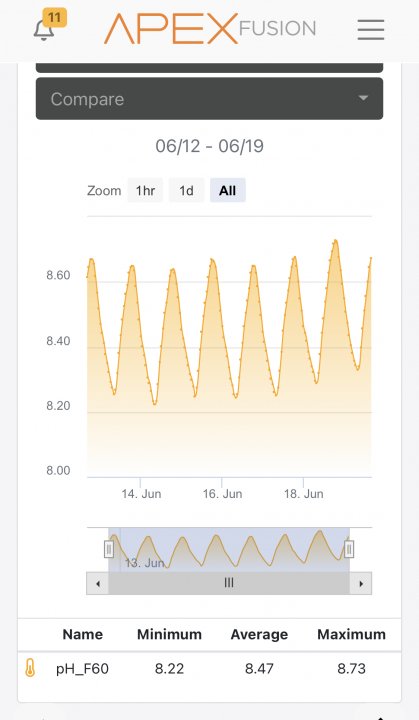
Oh no. I’m going to have to get my CO2 tanks refilled and start dosing based on pH again, or decrease light intensity for a bit in the afternoon to prevent what I guess is photosynthesis creating more O2 than can be removed (?) with aeration. I wish there was a way to program my light intensity to pH on Apex.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
What to do when pH is too high
- Thread starter Chromis
- Start date
If you haven’t already, I would re-calibrate your pH probe. My Apex pH probe imperceptibly drifts upward over the months. Your pH might not be as high as you think.
Your large swing in pH is telling you that aeration is insufficient to blunt the swing in pH caused by photosynthesis (during light hours) and metabolism (all the time but unopposed when lights are off). This is extremely common and the more living stuff you have in your tank the larger the swing. If you had over-the-top aeration there would be almost no swing in pH, it would be more based on CO2 levels in the room air, but in my opinion this probably is just theoretical unless you are willing to go to extremes. Which I have considered doing myself
Photosynthesis lowers dissolved CO2 = increased pH. Metabolism of every living thing in your tank increases dissolved CO2 = decreased pH. As Jim mentioned, it’s not the O2 causing this swing, it’s the CO2.
Lots of people intentionally run their system pH as high as yours so it might not actually be a problem. I personally like to target around 8.3 and don’t want to get higher than 8.5. But I absolutely would not change anything without recalibrating your probe first.
Your large swing in pH is telling you that aeration is insufficient to blunt the swing in pH caused by photosynthesis (during light hours) and metabolism (all the time but unopposed when lights are off). This is extremely common and the more living stuff you have in your tank the larger the swing. If you had over-the-top aeration there would be almost no swing in pH, it would be more based on CO2 levels in the room air, but in my opinion this probably is just theoretical unless you are willing to go to extremes. Which I have considered doing myself
Photosynthesis lowers dissolved CO2 = increased pH. Metabolism of every living thing in your tank increases dissolved CO2 = decreased pH. As Jim mentioned, it’s not the O2 causing this swing, it’s the CO2.
Lots of people intentionally run their system pH as high as yours so it might not actually be a problem. I personally like to target around 8.3 and don’t want to get higher than 8.5. But I absolutely would not change anything without recalibrating your probe first.
The fact that it drifted up when you changed something doesn’t mean the absolute values are correctly calibrated. If it’s reading 0.2 high at all points that would be important info.Ok so aeration isn’t the answer it’s injecting CO2 back in.
I’ll check the calibration also since I probably haven’t checked it in nearly a year but the pH has drifted back since I stopped dosing CO2.
Aeration might help. Normal outdoor CO2 of around 420 ppm results in pH of 8.3 with reef tank parameters. So the more you aerate, the more it will shift towards 8.3 (which is mostly down if your numbers are right). Or lower if your indoor CO2 level is higher than outdoors.
If you aren’t miscalibeated, you must be adding a lot of something that raises pH because photosynthesis alone wouldn’t raise your pH that high in a reef tank. Maybe in a tank with nothing but macroalgae. Are you using kalkwasser or sodium hydroxide for Alk? Adding less of whatever is pegging the pH high is what I’d do before adding CO2. After recalibrating
Not too crazy… in the past week it drifted up 0.2dKH.How is your Alk?
That’s what’s baffling. I don’t have a refugium although admittedly there’s like an 8”x8” sheet worth of ulva in the display part. I dose a alk mix of 2 parts baking soda to one part soda ash, primarily at night. I don’t dose any alk between 9a to 6p.The fact that it drifted up when you changed something doesn’t mean the absolute values are correctly calibrated. If it’s reading 0.2 high at all points that would be important info.
Aeration might help. Normal outdoor CO2 of around 420 ppm results in pH of 8.3 with reef tank parameters. So the more you aerate, the more it will shift towards 8.3 (which is mostly down if your numbers are right). Or lower if your indoor CO2 level is higher than outdoors.
If you aren’t miscalibeated, you must be adding a lot of something that raises pH because photosynthesis alone wouldn’t raise your pH that high in a reef tank. Maybe in a tank with nothing but macroalgae. Are you using kalkwasser or sodium hydroxide for Alk? Adding less of whatever is pegging the pH high is what I’d do before adding CO2. After recalibrating
I dose phosphate and nitrate into the tank. It’s a 5x2x12” frag tank (mostly acros) with 10” water column and good surface agitation. There’s 28” of fish in there.
I’m thinking about a tv show I watched with my kids where they explain the mass of trees is largely from carbon taken from air. Is CO2 also getting taken out of the water and sequestered into coral skeleton and does it make an impact on pH also?
The fact that it drifted up when you changed something doesn’t mean the absolute values are correctly calibrated. If it’s reading 0.2 high at all points that would be important info.
Aeration might help. Normal outdoor CO2 of around 420 ppm results in pH of 8.3 with reef tank parameters. So the more you aerate, the more it will shift towards 8.3 (which is mostly down if your numbers are right). Or lower if your indoor CO2 level is higher than outdoors.
If you aren’t miscalibeated, you must be adding a lot of something that raises pH because photosynthesis alone wouldn’t raise your pH that high in a reef tank. Maybe in a tank with nothing but macroalgae. Are you using kalkwasser or sodium hydroxide for Alk? Adding less of whatever is pegging the pH high is what I’d do before adding CO2. After recalibrating
I’d have to question that statement on photosynthesis not being the cause. If a tank is pretty grown over with large coral colonies to the brim, I could definitely see uptake of co2 during photosynthesis causing it to increase significantly. When I had 80+ colonies in the frag tank I was constantly going over 6.5-6.6 with a calibrated pH probe.
Hey this is really interesting, I've had issues on my alk dosing - if I dose soda ash, my alk gets too low unless I dose enough to have precip events, and if I dose baking soda my pH drops too low. Where'd you get the idea to mix them, and how is that going for you? How did you come to the 2:1 ratio?That’s what’s baffling. I don’t have a refugium although admittedly there’s like an 8”x8” sheet worth of ulva in the display part. I dose a alk mix of 2 parts baking soda to one part soda ash, primarily at night. I don’t dose any alk between 9a to 6p.
I dose phosphate and nitrate into the tank. It’s a 5x2x12” frag tank (mostly acros) with 10” water column and good surface agitation. There’s 28” of fish in there.
I’m thinking about a tv show I watched with my kids where they explain the mass of trees is largely from carbon taken from air. Is CO2 also getting taken out of the water and sequestered into coral skeleton and does it make an impact on pH also?
Also, wouldn't dosing just baking soda help with your high pH? So many questions lol
I just mix them according to pH considerations - how high or low the overall pH is and when I dose it. The 2:1 ratio is random, I’ve just been slowly decreasing the soda ash amount as the pH drifts up. I would just dose baking soda if I dosed during the day but since I dose counter to lights on, I don’t want to further decrease the night pH and increase the pH swing by using only baking soda.Hey this is really interesting, I've had issues on my alk dosing - if I dose soda ash, my alk gets too low unless I dose enough to have precip events, and if I dose baking soda my pH drops too low. Where'd you get the idea to mix them, and how is that going for you? How did you come to the 2:1 ratio?
Also, wouldn't dosing just baking soda help with your high pH? So many questions lol
@RandyC Why do you think you had high pH besides lots of acros? Was this a low detritus/bare bottom tank, how many fish did you have, and did you use a CO2 reactor or two part?
I also feel it’s the acros using up all the CO2 affecting the pH and injecting CO2 has helped growth just you’d expect in a freshwater planted tank.
I also feel it’s the acros using up all the CO2 affecting the pH and injecting CO2 has helped growth just you’d expect in a freshwater planted tank.
More flow where you dose. This shouldn't be an issue as everything should dissolve out when it hits the water.I dose soda ash, my alk gets too low unless I dose enough to have precip events
I agree, ph probe cal first. I wouldn't worry too much about 8.6 (or really ph at all) unless the coral looks unhappy. Is anything looking pale that normally is colored up?
I say just blow more bubbles in the tank instead of using a snorkel when doing the up close viewings. Or am I the only one who gets that close with my fish?
@RandyC Why do you think you had high pH besides lots of acros? Was this a low detritus/bare bottom tank, how many fish did you have, and did you use a CO2 reactor or two part?
I also feel it’s the acros using up all the CO2 affecting the pH and injecting CO2 has helped growth just you’d expect in a freshwater planted tank.
Bare bottom 6x3’ frag tank. It has 5 tangs and a fox face in it. The pH would peak at 3-4 hours after lights turned on and would fall fairly quickly soon after lights turned off. I was running calcium reactor and the tank was in the garage.
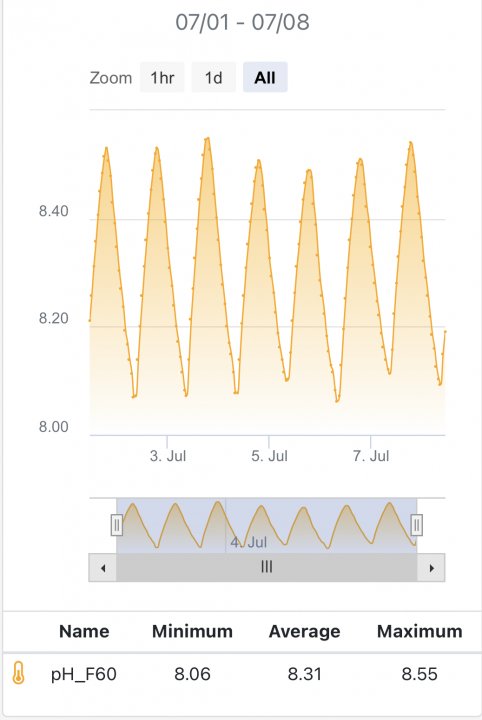
Just to follow up, recalibrated my pH probes and the pH isn’t quite as scary.
It took a couple weeks for the pH measurement to stabilize after calibration.
It had been exactly one year since the last calibration (using a BRS pH probe). The pH was off by something like .2
It took a couple weeks for the pH measurement to stabilize after calibration.
It had been exactly one year since the last calibration (using a BRS pH probe). The pH was off by something like .2
The pH probe is in a chamber before the return pump chamber, I dose alk and Ca into the return pump chamber, so the additives should have some time to mix in the display before getting back down to where the pH probe is in the sump.How close to the pH probe is anything you are dosing that impact pH? Otherwise, that is pretty much what my pH cycle looks like
If you care about accuracy, you need to clean your PH probe.
Crud builds up and messes with the measurements.
They have special cleaning solutions you soak the probe in.
https://milwaukeeinstruments.com/milwaukee-cleaning-solution-for-ph-orp-electrodes-230ml/
Now me ... I don't care that much. I usually occasionally brush it with soft toothbrush.
Crud builds up and messes with the measurements.
They have special cleaning solutions you soak the probe in.
https://milwaukeeinstruments.com/milwaukee-cleaning-solution-for-ph-orp-electrodes-230ml/
Now me ... I don't care that much. I usually occasionally brush it with soft toothbrush.

