Alexander1312
Supporting Member
Does anyone know exactly how to set up the Kalkwasser method he has been describing in various interviews? I recognize that not everyone supports this method, or feels comfortable with Alkalinity fluctuating a lot by focusing on PH only, but I would like to try this as I did see a strong correlation between the PH and how corals look, vs. relying on Alkalinity parameters. And the fact that the ocean appears to be stable at a PH of 8.3 seems convincing, if correct.
I would like to know which equipment is exactly needed since I am not too familar with the APEX system. I only bought the APEX Jr. some time ago, and the only thing it is doing in my tank is measuring PH and Temp.
I understand it needs the APEX dosing pump, and it looks as if the APEX Jr can handle the dosing pump, and I would not need another module.
- APEX Jr (or higher), including PH probe
- Apex DOS Dosing Pump & Fluid Metering System - Neptune Systems
- Kalkwasser - I will use the Captiv8 - 6.5g / gallon
- Kalkwasser container
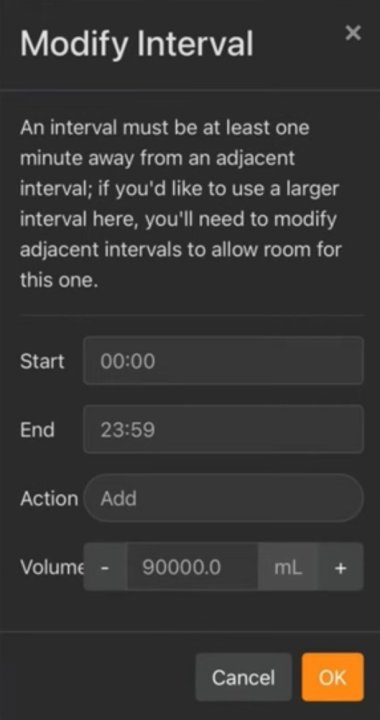
I understand then that the following needs to be 'programmed':
- Time period will be during lights-out period
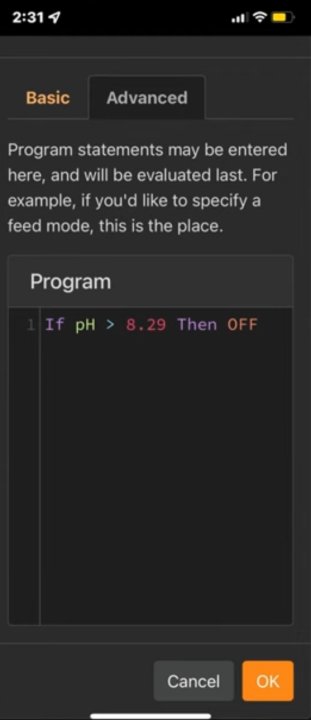
- Target PH will be the average PH for my tank when starting this method and then updated daily for continously higher average amounts until 8.29 average PH is reached.
- Evaporation will be the double amount of my daily evaporation during the half day 'on' time period
I have not used the programming of the APEX before but I assume this can be done with some research. Does anyone else have some adds to this, or has done this before, or notices any inaccuracies in what I am describing above?
It looks like mixing the kalkwasser with the right quantity, saturation and limited exposure to CO2 is key. Understanding the PH saturation of the solution seems to be essential (also mentioned in Kenny's video) so I have ordered the following PH probe with Temp adjustment:
- MW102 PH & Temperature Meter w/Auto Calc
I would like to know which equipment is exactly needed since I am not too familar with the APEX system. I only bought the APEX Jr. some time ago, and the only thing it is doing in my tank is measuring PH and Temp.
I understand it needs the APEX dosing pump, and it looks as if the APEX Jr can handle the dosing pump, and I would not need another module.
- APEX Jr (or higher), including PH probe
- Apex DOS Dosing Pump & Fluid Metering System - Neptune Systems
- Kalkwasser - I will use the Captiv8 - 6.5g / gallon
- Kalkwasser container
I understand then that the following needs to be 'programmed':
- Time period will be during lights-out period
- Target PH will be the average PH for my tank when starting this method and then updated daily for continously higher average amounts until 8.29 average PH is reached.
- Evaporation will be the double amount of my daily evaporation during the half day 'on' time period
I have not used the programming of the APEX before but I assume this can be done with some research. Does anyone else have some adds to this, or has done this before, or notices any inaccuracies in what I am describing above?
It looks like mixing the kalkwasser with the right quantity, saturation and limited exposure to CO2 is key. Understanding the PH saturation of the solution seems to be essential (also mentioned in Kenny's video) so I have ordered the following PH probe with Temp adjustment:
- MW102 PH & Temperature Meter w/Auto Calc

