I shall call him Lord Chonkulus.

You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
"The Lab" - Josh and Tiffany's IM Nuvo EXT 200
- Thread starter IOnceWasLegend
- Start date
Kensington Reefer
Supporting Member
Looks nice n thick
IMHO
fish with names tend to die first
i try not to name anything that doesn’t come when called
IMHO
fish with names tend to die first
i try not to name anything that doesn’t come when called
There’s only one fish I own that has a name and I adopted him with that name, lolLooks nice n thick
IMHO
fish with names tend to die first
i try not to name anything that doesn’t come when called
Wtf? Light in the tank, uggghhhhhAppreciate the offer!
And unfortunately, with the combination of the move and the light falling in the tank, the only torches I have left are dragon soul, hellfire, and dragon tamer.
Welp, that's unfortunate luck for us then since every fish in our tank has a name. (Minus the anthias group, which is just called 'the polymerase gang').Looks nice n thick
IMHO
fish with names tend to die first
i try not to name anything that doesn’t come when called
Yeah, wasn't fun at all. No idea how it happened since the screw that came loose was in a stud (I checked before, and re-checked afterwards). Cost us nearly every torch we had, including a nice malaysian gold.Wtf? Light in the tank, uggghhhhh
I've been losing about ~0.25 dkh of alkalinity a day, so I decided now was as good a time as any to fire up the calcium reactor (an Aquamaxx cTech T3 reactor, using an FX-STP2 peristaltic pump to pull fluid through, and CO2 rate controlled by a CarbonDoser). Given the minimal demand, I'm opting to keep the pH towards the higher end of the spectrum (CO2 on at 6.8, off at 6.6) under the assumption the slightly elevated pH will result in a slower breakdown/leaching of alk/Ca/Mg from the media.
Starting off with a feed rate of 30 mL/minute; and so far so good, I think?
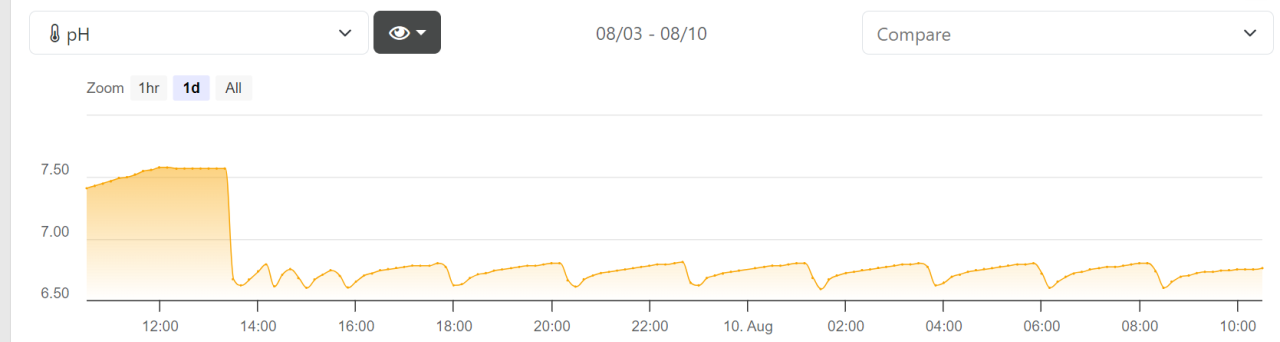
My only concern is that, even though I have the bubbles set on the slowest possible rate (1 bubble every 9-10 seconds), the pH drops extremely quickly; going from 6.8 to 6.6 in the span of ~20 minutes. My understanding is that the solenoid should be 'on' as much as possible, and the current setup means that it will undergo ~24 on/off events every day, or is this just not really a factor to worry about with electronic regulators?
Starting off with a feed rate of 30 mL/minute; and so far so good, I think?
My only concern is that, even though I have the bubbles set on the slowest possible rate (1 bubble every 9-10 seconds), the pH drops extremely quickly; going from 6.8 to 6.6 in the span of ~20 minutes. My understanding is that the solenoid should be 'on' as much as possible, and the current setup means that it will undergo ~24 on/off events every day, or is this just not really a factor to worry about with electronic regulators?
Kensington Reefer
Supporting Member
@IOnceWasLegend
how are the fish?
how are the fish?
Mostly good; still frustrating.@IOnceWasLegend
how are the fish?
Achilles has scratched a few times yesterday again, but nothing major. Keeping a close eye on him.
Could just be my imagination, but I feel like the tangs (except for the kole) + the foxface are breathing a bit fast. I just noticed that yesterday but I don't, however, have much of a baseline to make that call (plus I know they're the 'athletic' fish). I started the calcium reactor yesterday, so I'm wondering if that could be playing a role. Additionally, it's been about three weeks since I changed the carbon; just did a change today (possibility that, since I've been making/storing the water in a large container in the garage, there's some contaminant) and will keep monitoring.
They're all still eating, all still swimming fine, and all acting otherwise normally, though. Flukes are at the back of my mind again as a possibility, but - given that the fish breathing heavier have all been treated with prazipro in QT, and again in the tank - that's lower on the list of 'concerns over what could be going on' given the rarity of prazi-resistant flukes.
I've been losing about ~0.25 dkh of alkalinity a day, so I decided now was as good a time as any to fire up the calcium reactor (an Aquamaxx cTech T3 reactor, using an FX-STP2 peristaltic pump to pull fluid through, and CO2 rate controlled by a CarbonDoser). Given the minimal demand, I'm opting to keep the pH towards the higher end of the spectrum (CO2 on at 6.8, off at 6.6) under the assumption the slightly elevated pH will result in a slower breakdown/leaching of alk/Ca/Mg from the media.
Starting off with a feed rate of 30 mL/minute; and so far so good, I think?
View attachment 49126
My only concern is that, even though I have the bubbles set on the slowest possible rate (1 bubble every 9-10 seconds), the pH drops extremely quickly; going from 6.8 to 6.6 in the span of ~20 minutes. My understanding is that the solenoid should be 'on' as much as possible, and the current setup means that it will undergo ~24 on/off events every day, or is this just not really a factor to worry about with electronic regulators?
Have you adjusted the bubble size? I have the multiple older versions of the carbon doser regulator and it has the ability to adjust how much co2 is dropped (bubble size) per second.
Even though technically and a better implementation is having the exact rate of co2 dropped in without having to shut off the solenoid via pH probe, I’ve never bothered after years of using a co2 reactor and have never had crazy alk swings. I’ve managed everything through the pH probe and having trident to monitor and report the alk so I can see if anything is tending off course.
Sorry; meant to respond to this.Have you adjusted the bubble size? I have the multiple older versions of the carbon doser regulator and it has the ability to adjust how much co2 is dropped (bubble size) per second.
Even though technically and a better implementation is having the exact rate of co2 dropped in without having to shut off the solenoid via pH probe, I’ve never bothered after years of using a co2 reactor and have never had crazy alk swings. I’ve managed everything through the pH probe and having trident to monitor and report the alk so I can see if anything is tending off course.
I don't think my CarbonDoser allows me to adjust bubble size; secondary pressure is low (as low as I can get it) and the bubble rate is set at the slowest setting (10 seconds per bubble). I'm not too worried about alk swings, since I've got the CO2 regulator under the control of the pH probe (>6.8 on, <6.6 off) and it's oscillated between those two wonderfully.
My only concern was, like you said, having the proper rate of CO2 without having to shut off the solenoid (since it's currently taking ~15 minutes on to drop pH in the CaRx from 6.8 to 6.6, turning off, then about 2 hours to trickle back up to 6.8, repeat). If ~24 on-off cycles a day isn't going to cause issues, I'm fine with leaving it as-is since my alk has been stable.
And thanks for the input!
Update on the fish: apparently the hybrid achilles was just pissy that day (re: scratching a bit). I swapped the carbon out (and have set reminders to do that every 2 weeks) and, while I'm unsure if it was due to carbon or just the fish stressed for other reasons (picking on each other), the tangs' breathing is more normal now.
I also thought there was something wrong with one of the bimaculatus anthias because of some dark spots on its dorsal fin and tail, and then I realized it's started transitioning to male! I've been expecting this for a little while given it's the largest + how aggressive it's been to the other four, but still really exciting actually seeing it happen for the first time. And, speaking of exciting, the pod population has exploded so I can't wait to add the mandarin pair.
Finally, in spite of how it may appear, we're not planning on a FOWLR tank. As I noted earlier, we're pretty paranoid now re: biosecurity, so I'll be setting up a coral/invert QT system in the garage this week. Picked up the tank itself (IM Nuvo 14 peninsula) last week, and will be running it bare-bottom seeded with live rock from the holding tank's refugium.
The coral (and some inverts) in the 100g holding tank will be ready to add to the DT in mid-late September after a few rounds of Flatworm Exit. I'll have a decent amount of stuff to add to the QT system after the 9/10 frag swap (and picking some stuff up from @Invictus and @thephoreefer ). This will also give me time to source a fourth MP40, since I'd like to have one mounted to the back wall pointing towards the front for some cross/turbulent flow.
I also thought there was something wrong with one of the bimaculatus anthias because of some dark spots on its dorsal fin and tail, and then I realized it's started transitioning to male! I've been expecting this for a little while given it's the largest + how aggressive it's been to the other four, but still really exciting actually seeing it happen for the first time. And, speaking of exciting, the pod population has exploded so I can't wait to add the mandarin pair.
Finally, in spite of how it may appear, we're not planning on a FOWLR tank. As I noted earlier, we're pretty paranoid now re: biosecurity, so I'll be setting up a coral/invert QT system in the garage this week. Picked up the tank itself (IM Nuvo 14 peninsula) last week, and will be running it bare-bottom seeded with live rock from the holding tank's refugium.
The coral (and some inverts) in the 100g holding tank will be ready to add to the DT in mid-late September after a few rounds of Flatworm Exit. I'll have a decent amount of stuff to add to the QT system after the 9/10 frag swap (and picking some stuff up from @Invictus and @thephoreefer ). This will also give me time to source a fourth MP40, since I'd like to have one mounted to the back wall pointing towards the front for some cross/turbulent flow.
Kensington Reefer
Supporting Member
We'll be getting a porcupine puffer at some point in the next few months, but haven't added one yet.
Welp, I am now four for four of my display tanks getting dinoflagellates at some point. This time is lucky number 'small cell amphidinium'.
Blackout for a few days, running carbon, running skimmer, and dosing microbacter to maximize biodiversity it is.
Blackout for a few days, running carbon, running skimmer, and dosing microbacter to maximize biodiversity it is.
There’s only one fish I own that has a name and I adopted him with that name, lol
Would that be Doug?
Yes sir!!!!!Would that be Doug?
Welp, I am now four for four of my display tanks getting dinoflagellates at some point. This time is lucky number 'small cell amphidinium'.
Blackout for a few days, running carbon, running skimmer, and dosing microbacter to maximize biodiversity it is.
Oh man, I just read through your thread (awesome build!) and the whole time was wondering about dinos popping up...I had the exact same experience with a dry rock setup, and the dinos defeated me. Sounds like you've done your research since you've identified the type. I spent many months in the trenches with amphidinium, and gave up battling them after several months and rebooted.
None of these worked for me, but people have had success with: adding lots of pods, many frequent blackouts over an extended period of time, dosing silicates to help diatoms outcompete, cranking up nutrients and waiting several months, increasing temp to 83 degrees, dosing H2o2 over an extended period, seeding with live rock...or some combination of the above. The most consistent themes with success seem to be biodiversity and time.
Anyway I'll be following along and wishing you luck! Really sweet setup, and very well thought out....other than the lack of live rock!
Sorry to hear about your struggles, and appreciate the input. Fortunately, this isn't my first rodeo with dinoflagellates and I'm 3 for 3 in beating them so far. Also fortunate that these are small cell which, unlike large cell amphidinium, go into the water column during blackouts and are susceptible to UV.Oh man, I just read through your thread (awesome build!) and the whole time was wondering about dinos popping up...I had the exact same experience with a dry rock setup, and the dinos defeated me. Sounds like you've done your research since you've identified the type. I spent many months in the trenches with amphidinium, and gave up battling them after several months and rebooted.
None of these worked for me, but people have had success with: adding lots of pods, many frequent blackouts over an extended period of time, dosing silicates to help diatoms outcompete, cranking up nutrients and waiting several months, increasing temp to 83 degrees, dosing H2o2 over an extended period, seeding with live rock...or some combination of the above. The most consistent themes with success seem to be biodiversity and time.
Anyway I'll be following along and wishing you luck! Really sweet setup, and very well thought out....other than the lack of live rock!I've become a huge convert after this recent experience.
My strategy in the past has been increasing nutrients (if phosphates are zero or low), adding bacteria/biodiversity, and adding UV. So far, UV has been the most important piece of the puzzle
Sorry to hear about your struggles, and appreciate the input. Fortunately, this isn't my first rodeo with dinoflagellates and I'm 3 for 3 in beating them so far. Also fortunate that these are small cell which, unlike large cell amphidinium, go into the water column during blackouts and are susceptible to UV.
My strategy in the past has been increasing nutrients (if phosphates are zero or low), adding bacteria/biodiversity, and adding UV. So far, UV has been the most important piece of the puzzle
Ahhh yeah, UV pretty much trivializes the ones that enter the water column. My version of amphidinium did not, unfortunately!
